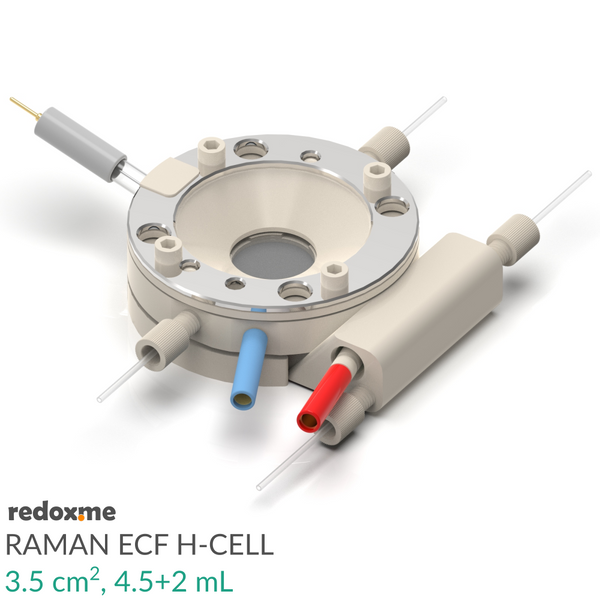
Raman ECF H-Cell 3.5 cm2, 4.5+2 mL – Raman Electrochemical Flow H-Cell, active area: 3.5 cm2, volume: 4.5+2 mL
This cell combines two classical analytical techniques such as electrochemistry and Raman spectroscopy, to obtain in-situ chemical information about the reactions taking place during an electrochemical experiment. The Raman Electrochemical Flow H-Cell offers several advantages over a single-compartment Raman Electrochemical Flow Cell. First of all the ion-exchange membrane allows for the physical separation of the anode and cathode compartments. This prevents direct mixing of the reactants and products, which is essential for studying specific electrode reactions without interference. Each compartment in the cell can have different electrolytes or pH conditions, allowing for precise control over the reaction environment. This control is crucial for investigating the influence of different factors on electrocatalysis, such as pH, ionic strength, and the presence of specific ions. In a single-compartment cell, unwanted side reactions can occur due to the direct interaction of reactants and products at the electrode surface. With an ion-exchange membrane separating the compartments in the Raman Electrochemical Flow H-Cell cross-talk between the anode and cathode reactions is minimized, leading to more accurate measurements of electrocatalytic activity. Certain species present in one compartment of the cell can interfere with the electrocatalytic reaction occurring at the electrode surface in a single-compartment cell. In the Raman Electrochemical Flow H-Cell the ion-exchange membrane acts as a barrier, preventing such interferences from reaching the electrode surface and affecting the electrocatalytic process. Moreover, Raman Electrochemical Flow H-Cell with ion-exchange membrane exhibits greater stability over time compared to single-compartment cells. The membrane helps maintain the integrity of the cell by preventing mixing of electrolytes and minimizing the likelihood of electrode fouling or degradation.
It consists mainly of four elements: (i) sample holder with Tantalum foil serving as an electric contact to working electrode surface, (ii) working electrode compartment in which reference is installed together with electrolyte inlet and outlet, (iii) lid which seals the cell and holds the Sapphire window, and (iv) counter electrode compartment which holds the ion-exchange membrane and where the counter electrode is installed.
The sample consisting of a thin film of electrochemically active material deposited on a rigid or flexible substrate (working electrode) is loaded from the bottom via magnetic or screw mount. Both 2-, and 3-electrode setup is feasible.
During an experiment Raman laser is focused onto the surface of a thin film working electrode through a transparent Sapphire window and thin layer of electrolyte (total optical path of 3.25 mm).
The electrode adapter for installing 6 mm dia. electrodes inside Raman Electrochemical Flow H-Cell is available here. It enables using disk electrode, plug with clip or any other rod-shape electrode (e.g. graphite rod) or current collector (e.g. metal mesh, graphite coated metal mesh, metal foil, graphite coated metal foil, metal foam, carbon woven and non-woven fabrics, carbon paper, etc.) as working electrode instead of a flat electrode in default configuration.
The Raman Electrochemical Flow H-Cell can be converted into GDE Raman ECF H-Cell by using Gas Compartment. This conversion allows the installation of Gas Diffusion Electrodes as the working electrodes. This solution is available on request.
The cell elements are constructed with materials that are inert to the sample (PEEK, Fluorocarbons). It well fits aqueous (FKM O-Rings) and organic solvent (FFKM O-Rings) electrolyte requirements. The construction is gas-tight and can be used when the removal and exclusion of contaminants such as oxygen and water is required by bubbling of an inert gas through the electrolyte (in an external reservoir).
Application note
This cell can be used to track kinetic phenomena such as the near-surface proton concentration changes during oxidation and reduction reaction at working electrode. It can be also used to identify materials such as carbon, metal oxides, polymers and electrolytes, and to determine their structure and distribution. Various metals are suitable for this cell as auxiliary electrode including Platinum, Gold and Silver.
The use of Raman Electrochemical Flow H-Cell with an ion-exchange membrane provides a more controlled and reliable platform for studying electrocatalysis, allowing researchers to investigate fundamental mechanisms and optimize catalytic performance with greater precision. For troubleshooting instruction see the redox.me blog post
Specification
nominal exposure area: 3.5 cm2
electrolyte volume: 4.5 mL (WE) + 2 mL (CE)
optical path (including Sapphire window): 3.25 mm
electrode plug diameter: 6 mm
membrane size: 20 mm dia.
Intrastat data
HS Code: 90275000
Country of Origin: Sweden
NET weight: 200g
Product includes
1 x working electrode compartment
1 x Ag/AgCl or non-aqueous Ag/Ag+ 30 mm reference electrode
1 x Metal wire electrode 35HX15 0.6/235 MM, Platinum
1 x Sapphire window
1 x lid
1 x lid pressing metal frame
1 x WE Tantalum contact
1 x sample holder
1 x counter electrode compartment
1 x plug
Related products
BEC 50 mL - Basic Electrochemical Cell
Gas Compartment
Peristaltic pump