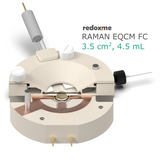
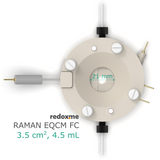
Raman EQCM FC 3.5 cm2, 4.5 mL – Raman Electrochemical Quartz Crystal Microbalance Flow Cell Flow Cell

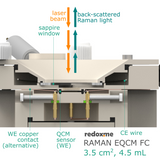
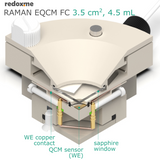
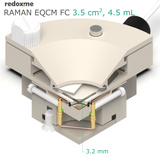
This cell combines three classical analytical techniques such as electrochemistry, Raman spectroscopy and Quartz Crystal Microbalance, to obtain in-situ chemical information about the reactions taking place during an electrochemical experiment. It consists mainly of three elements: (i) QCM sensor holder with gold plated pogo pins to access sensor electrode contacts placed on a bottom as well as a top surface; (ii) chamber in which reference and counter electrodes are installed together with electrolyte inlet and outlet and (iii) lid which seals the cell. This cell works with both standard sizes of quartz sensors, either 14 mm or 25.4 mm in diameter. In this configuration, a thin film of measured material is deposited directly onto upper electrode of a QCM sensor or the material is dispersed in an electrolyte. The counter or/and reference electrodes are mounted in a top casing (either 2-, or 3-electrode setup. The oval counter electrode made of e.g. Platinum wire assures uniform distribution of the field lines along the path to working electrode. During an experiment Raman laser is focused onto the surface of a thin film working electrode through a transparent Sapphire window and thin layer of electrolyte (total optical path of 3.2 mm). The electrolyte thickness of 2.2 mm ensures free diffusion of ions (e.g. protons) and its counter ions.
The cell elements are constructed with materials that are inert to the sample (glass and PEEK). It well fits aqueous (FKM/EPDM O-Rings) and organic solvent (FFKM O-Rings) electrolyte requirements. The construction is gas-tight and can be used when the removal and exclusion of contaminants such as oxygen and water is required by bubbling of an inert gas through the electrolyte (in an external reservoir).
Application note
This cell can be used to track kinetic phenomena such as the near-surface proton concentration changes during oxidation and reduction reaction at working electrode. At the same time EQCM allows studying changes in the interfacial mass and physical properties associated with electron transfer processes occurring at the electrode surface, such as those accompanying electropolymerization of thin films. It can be also used to identify materials such as carbon, metal oxides, polymers and electrolytes, and to determine their structure and distribution. Various metals are suitable for this cell as auxiliary electrode including Platinum, Gold and Silver. For troubleshooting instruction see the redox.me blog post
Specification
compatibility: standard QCM sensor with 14 mm or 25.4 mm dia.
electrolyte volume: 4.5 mL
optical path (including Sapphire window): 3.20 mm
electrode plug diameter: 6 mm
Intrastat data
HS Code: 90160090
Country of Origin: Sweden
NET weight: 100g
Product includes
1 x chamber
1 x Ag/AgCl or non-aqueous Ag/Ag+ 30 mm reference electrode
1 x Metal wire auxiliary electrode – ST 0.6/150 mm, Platinum
1 x Sapphire window
1 x lid
1 x frame holding the lid
1 x WE upper copper contact
1 x QCM sensor holder
1 x plug
Related products
BEC 50 mL - Basic Electrochemical Cell
Magnetic Mount Raman Electrochemical Flow Cell