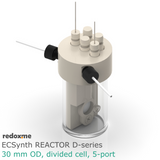
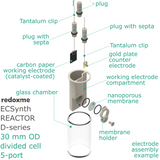
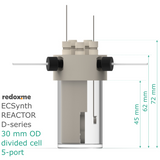
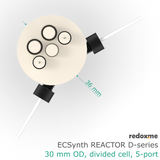
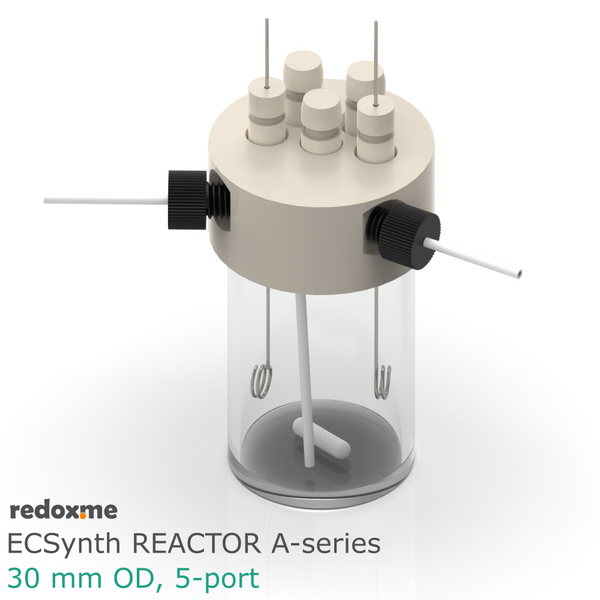
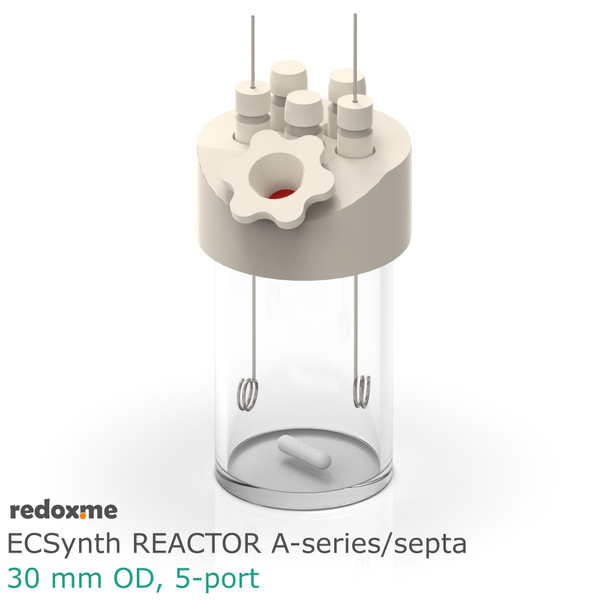
Electrosynthesis Reactor D-series, 30 mm OD, divided cell, 5-port

Electrosynthesis Reactor D-series is a divided batch cell that contains a solution of the substrates and reagents and it is operating for the time needed to complete the desired reaction until all the starting material in working electrode compartment is consumed. The electrolyte in working electrode compartment is separated from the rest of the cell by ion-exchange membrane or nanoporous membrane (13 mm dia.). The gas headspace above working and counter electrode is shared. The reaction mixture can be stirred using a magnetic stirrer to have a homogeneous concentration throughout the working electrode compartment.
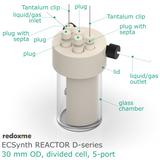
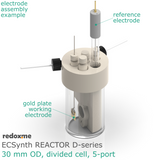
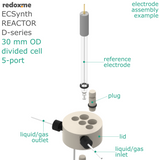
In this reactor, a solid anode (positive) and cathode (negative) are installed in Tantalum clips. If 3-electrode setup is required, the reference electrode is mounted in the reactor lid above working electrode compartment. Such electrode assembly is introduced into a solution containing the substrate and a supporting electrolyte, in order to carry the current through the cell separated by the membrane.
The reactor can be heated up or cooled down according to the requirements of the reaction.
The reactor elements are constructed with materials that are inert to most of the aqueous/organic reaction environments (glass, PEEK, PTFE tubing, FKM/FFKM O-Rings). The construction of this reactor is liquid- and gas-tight.
Application note
This electrosynthesis reactor is suitable for both, constant current and constant potential electrolysis in aqueous or organic media. As this is a divided cell, it can be used when the substrate and product formed could react at the counter electrode, or with the products produced as a counter reaction.
Various anode materials are suitable for this reactor including: (i) noble metals such as platinum, platinum metals or their alloys, and gold, (ii) carbon allotropes such as graphite, reticulated vitreous carbon or glassy carbon, (iii) Boron-Doped Diamond plate and thin film on a substrate, and (iv) other metals such as nickel, lead or lead oxide.
As the corrosive effects are not common in cathode materials, there is a wider variety of materials to choose including: (i) Boron-Doped Diamond plate and thin film on a substrate, (ii) platinum plate or wire and (iii) other metals such as copper, nickel, stainless steel, lead and tin.
Concerning the form of the anode and the cathode, the materials with high surface-area-to-volume ratio are preferential. Copper and nickel are usually used in a form of a foam, while the other metals are in a form of plates, meshes and wires.
Typical reference electrodes include Ag/Ag+ RE (organic media), Ag/AgCl (aqueous media) and pseudo-RE (Pt, Au, Ag).
None of the above mentioned electrodes is included in the reactor, so they need to be added to the quotation separately. For troubleshooting instruction see the redox.me blog post
Specification
maximum electrolyte volume at WE: 8mL
maximum electrolyte volume at CE: 10 mL
minimum electrolyte volume at WE: 2mL
minimum electrolyte volume at CE: 4 mL
electrode plug diameter: 6 mm
number of electrode slots: 5
membrane diameter: 13 mm
distance between anode and cathode: 18 mm
outer diameter of the glass chamber: 30 mm
effective height of the glass chamber: 45 mm
maximum operational temperature: 200 deg C
minimum operational temperature: -20 deg C
tubbing size: 1 mm ID, 1.6 mm OD
Intrastat data
HS Code: 90309000
Country of Origin: Sweden
NET weight: 100g
Product includes
1 x lid
1 x working electrode compartment
1 x membrane holder with a PEEK screw
1 x glass chamber
3 x plug
2 x plug with septum
10 x Tantalum clips
1 x set of O-Rings
2 x flangeless nuts for gas intel/outlet (1/16’’, 1.6 mm tubing)
2 x flangeless ferrules (1/16’’, 1.6 mm tubing)
Related products
Boron-Doped Diamond Plates
Noble Metal Plates
Glassy Carbon Plates
Tantalum clips
Reference Electrodes
Current Collectors